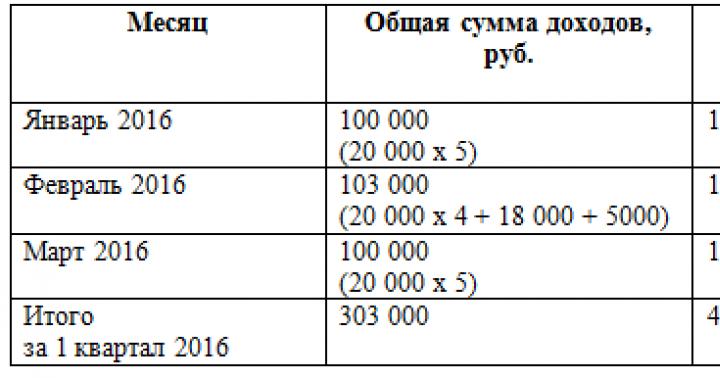
Today we will conduct a lesson not only in modeling, but also in chemistry, and we will make models of molecules from plasticine. Plasticine balls can be imagined as atoms, and ordinary matches or toothpicks will help to show structural connections. This method can be used by teachers when explaining new material in chemistry, and by parents when checking and studying homework and the children themselves, who are interested in the subject. Easier and accessible way creating visual material for mental visualization of micro-objects is, perhaps, impossible to find.
Here are representatives from the world of organic and inorganic chemistry as examples. By analogy with them, other structures can be made, the main thing is to understand all this diversity.
Materials for work:
- plasticine of two or more colors;
- structural formulas of molecules from the textbook (if necessary);
- matches or toothpicks.
1. Prepare plasticine for modeling spherical atoms from which molecules will be formed, as well as matches to represent the bonds between them. Naturally, it is better to show atoms of different types in a different color, so that it is clearer to imagine a specific object in the microworld.
2. To make balls, pinch off the required number of portions of plasticine, knead in your hands and roll into shapes in your palms. To sculpt organic hydrocarbon molecules, you can use larger red balls - this will be carbon, and smaller blue balls - hydrogen.

3. To form a methane molecule, insert four matches into the red ball so that they point towards the vertices of the tetrahedron.

4. Place blue balls on the free ends of the matches. The natural gas molecule is ready.

5. Prepare two identical molecules to explain to your child how the molecule of the next hydrocarbon, ethane, can be obtained.

6. Connect the two models by removing one match and two blue balls. Ethan is ready.

7. Next, continue the exciting activity and explain how a multiple bond is formed. Remove the two blue balls and make the bond between the carbons double. In a similar way, you can mold all the hydrocarbon molecules necessary for the lesson.

8. The same method is suitable for sculpting molecules of the inorganic world. The same plasticine balls will help you realize your plans.

9. Take the central carbon atom - the red ball. Insert two matches into it, defining the linear shape of the molecule; attach two blue balls, which in this case represent oxygen atoms, to the free ends of the matches. Thus, we have a carbon dioxide molecule of linear structure.

10. Water is a polar liquid, and its molecules are angular formations. They consist of one oxygen atom and two hydrogen atoms. The angular structure is determined by the lone pair of electrons on the central atom. It can also be depicted as two green dots.

These are the kind of exciting creative lessons you should definitely practice with your children. Students of any age will become interested in chemistry and will understand the subject better if, during the learning process, they are provided with a visual aid made by themselves.

organic chemistry molecule isology
It is now generally accepted that one straight line connecting two atoms represents one two-electron bond ( simple connection), the formation of which requires one valence from each of the bonded atoms, two lines - one four-electron bond (double bond), three lines - one six-electron bond (triple bond).
A representation of a compound with a known order of bonds between all atoms using bonds of this type is called a structural formula:
To save time and space, abbreviated formulas are often used, in which some of the connections are implied but not written:
Sometimes, especially in carbocyclic and heterocyclic series, the formulas are further simplified: not only are some bonds not written, but also some of the carbon and hydrogen atoms are not depicted, but are only implied (at the intersections of the lines); simplified formulas:
Tetrahedral model of the carbon atom
Basic ideas about chemical structure, laid down by A. M. Butlerov, were supplemented by Van't Hoff and Le Bel (1874), who developed the idea of the spatial arrangement of atoms in the molecule of an organic substance and raised the question of the spatial configuration and conformation of molecules. Van't Hoff's work “Chemistry in Space” (1874) marked the beginning of a fruitful direction in organic chemistry - stereochemistry, i.e. the study of spatial structure.
Rice. 1 - Van't Hoff models: methane (a), ethane (b), ethylene (c) and acetylene (d)
Van't Hoff proposed a tetrahedral model of the carbon atom. According to this theory, the four valencies of the carbon atom in methane are directed towards the four corners of the tetrahedron, in the center of which there is a carbon atom, and at the vertices are hydrogen atoms (a). Ethane, according to Van't Hoff, can be imagined as two tetrahedrons connected at the vertices and freely rotating about a common axis (6). The model of the ethylene molecule represents two tetrahedra connected by edges (c), and molecules with a triple bond are represented by a model in which the tetrahedra are in contact with planes (d).
Models of this type have also proven to be very successful for complex molecules. They are successfully used today to explain a number of stereochemical questions. The theory proposed by van't Hoff, although suitable in almost all cases, did not, however, provide a reasonable explanation of the type and essence of binding forces in molecules.
An innovative way to develop technology for creating new medicines
First, a computer model of the object is created, and computer modeling is also used to form molecules at the research site. The model can be either two-dimensional or three-dimensional...
Infrared spectra of molecules
In contrast to the visible and ultraviolet ranges, which are caused mainly by electron transitions from one stationary state to another...
Study of the structure of organic compounds using physical methods
All possible positions of molecules in three-dimensional space are reduced to translational, rotational and vibrational motion. A molecule consisting of N atoms has only 3N degrees of freedom of movement...
Modeling method in chemistry
Currently, you can find many different definitions of the concepts “model” and “simulation”. Let's look at some of them. “A model is understood as a representation of facts, things and relationships of a certain field of knowledge in the form of a simpler...
Scientific foundations of rheology
The stress-strain state of a body is generally three-dimensional and it is unrealistic to describe its properties using simple models. However, in those rare cases when uniaxial bodies are deformed...
In addition to observation and experiment, modeling plays an important role in understanding the natural world and chemistry. One of the main goals of observation is to search for patterns in the results of experiments...
Dissolution of solids
For the vast majority of processes, the kinetic function is invariant with respect to the concentration of the active reagent and temperature. In other words, each value of dimensionless time x corresponds to a very specific value...
Calculation of quantum chemical parameters of PAS and determination of the structure-activity relationship using the example of sulfonamides
Refractometric method of analysis in chemistry
Synthesis and analysis of chemical substances in gasoline production
The chemical model of the catalytic cracking process is very complex. Let's consider the simplest of the reactions occurring during the cracking process: CnH2n+2 > CmH2m+2 + CpH2p...
Synthesis of chemical technological system (CTS)
Production processes varied in their characteristics and degree of complexity. If the process is complex and deciphering its mechanism requires a lot of effort and time, an empirical approach is used. Mathematical models...
Comparison of plug-flow and full-mix reactors in isothermal operating mode
7.1. The figure shows an experiment illustrating that bodies expand when heated. With a pen, circle in the picture the object that was heated in this experiment - a ball or a ring. Justify your answer.
7.2. Choose the correct statement.
According to modern ideas, when a flask with water cools, the water level in the tube drops because... . 
7.3. Substances are made up of tiny particles. What phenomena and experiments confirm this?
7.4. The table shows exact data on the change in the volume of water V as a function of time t during heating.
Answer the questions.
a) Is it possible to say that during the entire observation time the water in the flask was heated evenly? Explain your answer.
b) How did the volume of water change when heated?

8.1. Choose the correct statement.
If you heat a nail, it lengthens and becomes thicker. This happens because when heated... . 
8.2. Write the words molecule, drop, atom in such an order that each subsequent element is part of the previous one.
8.3. The figure shows models of molecules of water, oxygen and carbon dioxide. All molecules contain an oxygen atom (black). Fill in the blanks in the text.

8.4. Measure the length of your arm from your elbow to your little finger and compare the measurement to the size of a water molecule.
9.1. Fill in the blanks in the text. “In ____, the English botanist Robert Brown, looking through a microscope...”

9.2. The figure schematically shows liquid molecules surrounding a grain of paint placed in this liquid. The arrows indicate the direction of movement of liquid molecules at a certain point in time.

9.3. Mark those phenomena that are examples of Brownian motion.
9.4. The figure shows a broken line along which a speck of dust moved in the air for several seconds.

a) Explain why the speck of dust changed the direction of its movement many times during the observation of it.
Due to collisions with air molecules and other dust particles.
b) In the figure, indicate the points at which the dust particle was affected by the molecules surrounding it.
10.1. Clean water is poured into a glass cylinder from above, and a solution is poured into the bottom through a narrow tube. copper sulfate. The cylinder is at rest when constant temperature. Show in the figure what the contents of the cylinder will look like at various intervals.

10.2. Two identical rubber balls are connected by a transparent hose (see figure), and the left ball in both cases is filled with hydrogen (color the hydrogen blue), the right one is empty in figure a, and is filled with air in figure b (color the air green). The hose is clamped between the balls.

10.3. Cross out one of the highlighted words to complete the correct explanation of the experiment described.

10.4. Home experiment.
Place at the bottom of a glass with cold water lump of sugar, but do not stir. Write down how long it took you to detect the presence of sugar molecules on the surface of the water in the glass and what “device” you used.
11.1. Fill in the gaps in the text using the words: stronger; weaker; attraction; repulsion.

11.2. Connect the phenomena and their corresponding explanations with lines.

11.3. Cross out one of the highlighted words to complete the correct explanation of the experiment described.

11.4. Complete the sentence to get the correct explanation of the phenomenon.
11.5. Fill in the blanks in the text. “In everyday life, we often encounter the phenomena of wetting and non-wetting.”

12.1. What state of matter is characterized by the listed characteristics?
GBPOU NSO "Kolyvan Agrarian College"
Instructional technological map No. 1
according to OUD. 11 Chemistry
professions 35.01.23 Mistress of the estate, 01/19/04 Baker
Section 1: Organic Chemistry
Topic 1.1: Basic concepts of organic chemistry and the theory of the structure of organic compounds.
Job title : Making models of molecules - representatives of various classes of organic compounds.
Purpose of the work:
generalize and systematize students’ knowledge about the theory of the structure of organic compounds;
consolidate the ability to compose structural formulas of hydrocarbons;
The student must achieve the following results:
personal:
− a sense of pride and respect for the history and achievements of domestic chemical science; chemically competent behavior in professional activities and at home when handling chemicals, materials and processes;
− readiness to continue education and advanced training in the chosen professional activity and objective awareness of the role of chemical competencies in this;
− the ability to use the achievements of modern chemical science and chemical technologies to improve one’s own intellectual development in the chosen professional activity;
meta-subject:
− usage various types cognitive activity and basic intellectual operations (statement of the problem, formulation of hypotheses, analysis and synthesis, comparison, generalization, systematization, identification of cause-and-effect relationships, search for analogues, formulation of conclusions) to solve the problem, the use of basic methods of cognition (observation, scientific experiment) to study various aspects of chemical objects and processes that need to be encountered in the professional field;
− the use of various sources to obtain chemical information, the ability to assess its reliability in order to achieve good results in the professional field;
subject :
− the formation of ideas about the place of chemistry in the modern scientific picture of the world;
Understanding the role of chemistry in shaping a person’s horizons and functional literacy for solving practical problems;
− mastery of fundamental chemical concepts, theories, laws and patterns;
Confident use of chemical terminology and symbols;
− mastery of the basic methods of scientific knowledge used in chemistry: observation, description, measurement, experiment;
Ability to process, explain the results of experiments and draw conclusions;
− willingness and ability to apply cognitive methods in solving practical problems;
− developed ability to give quantitative estimates and make calculations using chemical formulas and equations;
− knowledge of safety rules when using chemicals;
− formation of one’s own position in relation to chemical information obtained from various sources.
Form of study : individual
Standard time: 2 hours
Workplace equipment : Set of ball-and-stick models of molecules, table “Saturated hydrocarbons”, periodic table, instructions technological maps, notebooks
Literature:
Learning Tools: verbal (verbal), visual
Safety precautions: familiarized with safety rules at the workplace and in the office.
Guidelines
Hydrocarbons are organic substances consisting of carbon and hydrogen atoms. The carbon atom in all organic compounds is tetravalent. Carbon atoms can form straight, branched, and closed chains. The properties of substances depend not only on the qualitative and quantitative composition, but also on the order of connection of atoms with each other. Substances that have the same molecular formula, but different structures are called isomers. Prefixes indicate quantitydi - two,three - three,tetra - four;cyclo - means closed.
Suffixes in the names of hydrocarbons indicate the presence of a multiple bond:
en
single bond between carbon atoms(C - C);
en
double bond between carbon atoms(C = C);
in
triple bond between carbon atoms(WITH
=
WITH);
diene
two double bonds between carbon atoms(C = C - C = C);
Radicals:methyl -CH 3 ; ethyl -C 2 N 5 ; chlorine -Cl; bromine -Br.
Example. Make a model of a propane molecule.
Propane moleculeC 3 H 8 contains three carbon atoms and eight hydrogen atoms. The carbon atoms are connected to each other. Suffix– en indicates the presence of a single bond between carbon atoms. Carbon atoms are located at an angle of 109 28 minutes.
The molecule has the shape of a pyramid. Draw carbon atoms as black circles, hydrogen atoms as white circles, and chlorine atoms as green circles.
When drawing models, observe the ratio of atomic sizes.
Find the molar mass using the periodic table
M(S 3 N 8 ) = 12 · 3 + 1 · 8 = 44 g/mol.
To name a hydrocarbon you need to:
Choose the longest chain.
Number starting from the edge to which the radical or multiple bond is closest.
Indicate the radical if several radicals are indicated each. (Number before the name).
Name the radical, starting with the smallest radical.
Name the longest chain.
Indicate the position of the multiple bond. (Number after name).
Example

When composing formulas by name, you need to:
Determine the number of carbon atoms in the chain.
Determine the position of the multiple bond. (Number after name).
Determine the position of radicals. (Number before the name).
Write down the formulas of radicals.
Lastly, determine the number and arrangement of hydrogen atoms.
Work order
Task No. 1 . Make models of molecules:
1) a number of alkanes: methane, ethane, butane, pentane, hexane, heptane, octane, nonane and decane;
2) Cycloalkanes: cyclopropane,cyclopetane
3) 2-methylpropane,
4) 1,2-dichloroethane.
Draw molecular models in your notebook. Write the structural formulas of these substances. Find their molecular weights.
Task No. 2. Name the substances:

Task No. 3. Compose structural formulas of substances:
a) butene-2, write its isomer;
b) 3,3 - dimethylpentine-1.
What is the general formula for saturated hydrocarbons?
Which substances are called homologs and which are isomers?
Teacher: Rachkovskaya A.I.